Diabetes Management with Tandem Mobi
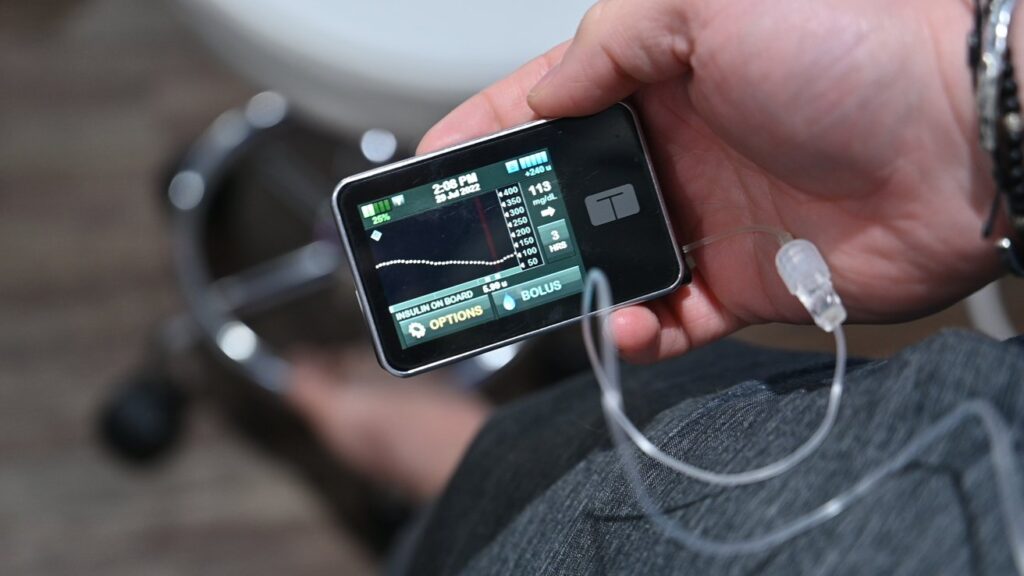
Tandem Diabetes Care has achieved a groundbreaking milestone with the FDA clearance of its innovative Mobi automated insulin delivery system. Positioned as the world’s smallest insulin delivery system fully controllable by a smartphone, the Tandem Mobi introduces a new era in diabetes management.
Tip: Fill out this form to check if you or a friend qualify for CGM Devices.
Expanded Access for Pediatric Diabetes
Tandem Diabetes Care recently announced an expansion of the age range for the Tandem Mobi, making it available for children aged 2 and older with type 1 diabetes. This development follows the earlier FDA clearance for individuals aged 6 and up, providing families and healthcare teams with a powerful tool to navigate the complexities of pediatric diabetes.
Control-IQ Technology: A Game-Changer
At the heart of the Tandem Mobi is the revolutionary Control-IQ technology, a prescription-only software designed to automate blood sugar control. By analyzing continuous glucose monitor (CGM) readings, the system intelligently determines the optimal insulin delivery, fostering better glucose management.
Must Read CGMs in noncritical care hospitals optimizes glycemic control
Compact Design and Mobile Control
Setting a new standard in portability, the Tandem Mobi claims the title of the smallest durable automated insulin delivery system controllable through a mobile app. With a size less than half that of the t:slim X2, Tandem’s current hybrid closed-loop system, the Mobi includes a 5-inch tubing option. While lacking a screen, the Mobi offers full iOS mobile control through an iPhone app, featuring an on-pump button for convenient manual bolusing.
Wearability Options and Water Resistance
The Tandem Mobi insulin pump, despite its small size, holds a 200-unit insulin cartridge and fits comfortably in a coin pocket. Users can choose from various wearability options, such as clipping it to clothing or utilizing an armband or adhesive sleeve. Notably, the Mobi is water-resistant, enhancing its durability in various lifestyle scenarios.
Read Guide about Wegovy Dosage Guide: The Best Way For Weight Loss
Advanced Features and Connectivity
The Tandem Mobi isn’t just compact; it’s a feature-packed device. It supports wireless charging and can receive remote software updates, ensuring users benefit from the latest advancements. The FDA clearance positions Tandem to directly compete with Insulet’s Omnipod 5, presenting a compelling choice in the automated insulin delivery landscape.
Also, read about Eli Lilly’s Breakthrough Obesity Treatment
Future Integrations and Limited Release
Presently compatible only with Dexcom CGM, Tandem Diabetes Care is actively working to incorporate Abbott’s FreeStyle Libre into its technology. The company anticipates a limited release of the Tandem Mobi in fall 2023, with plans for full FDA approval in 2024. Prospective users can subscribe to receive updates on the Mobi system’s availability through Tandem’s official website.
Conclusion
In conclusion, Tandem Mobi emerges as a game-changer, offering a sophisticated and user-friendly approach to diabetes management. With its compact design, advanced technology, and upcoming integrations, the Mobi is poised to redefine the landscape of automated insulin delivery systems. Stay tuned for the future of diabetes care with Tandem Mobi.