Eversense 365, the newest implantable continuous glucose monitoring (CGM) system for adults with diabetes that can last up to a year, has received FDA approval. Without a replacement, the Eversense E3 monitor from before could last up to six months.
A tiny sensor, roughly the size of a grain of rice, is implanted beneath the skin of the upper arm in the Eversense 365 implant. Patients can track their blood sugar levels in real-time by using a smartphone app that receives data from the sensor every five minutes.
“Eversense 365’s clearance marks a major advancement in Continuous Glucose Monitor (CGM) technology. Tim Goodnow, PhD, president and CEO of Senseonics, stated in a statement that “extending sensor longevity to a full year, coupled with seamless device connectivity and a high level of accuracy, provides both freedom and peace of mind to patients living with diabetes.”
Details About CGM Operation, Possible Side Effects, And Availability
Here are some further details about Continuous Glucose Monitor (CGM) operation, possible side effects, and availability
How Does Eversense 365 Work?
According to Brian Hansen, president of Continuous Glucose Monitor (CGM) at Ascensia Diabetes Care, inserting the Eversense 365 sensor is a rapid in-office process that takes around five minutes for a qualified healthcare professional.
Unlike many other devices that require more frequent replacements, once installed, the sensor can monitor your blood sugar levels for up to a year with little to no disruptions.
Fingerstick measurements are still necessary for calibration, but after the first two weeks (day 13), the Eversense system only needs them once a week, which is practical for consumers and gives them confidence in their glucose readings, according to Hansen.
The device’s “unique, fully implantable design helps people overcome common frustrations experienced with traditional short-term Continuous Glucose Monitors (CGMs), like not wearing the device for the recommended amount of time, falling off, skin irritation from harsh adhesives, and false alerts at night,” according to Hansen.
According to Hansen, another feature of Eversense 365 is a detachable transmitter that you may remove when you’re done receiving glycemic data updates. “Users experience maximum comfort and almost no skin reactions because the transmitter is placed over the sensor on the upper arm with a gentle, silicone-based adhesive that is changed daily,” he explained.
Hansen stated that certain persons in the United States will still be able to purchase the previous model, the Eversense E3. “These are both fully implantable, long-term continuous glucose monitors that enable individuals with diabetes to lead normal lives,” the speaker stated.
Wearable Tech to Manage Your Diabetes
What Is the Eversense CGM’s Accuracy?
According to a study on the reliability and safety of the Eversense CGM System, the sensor lasted 97% of the time during 365 days and was safe, with only a few cases of mild skin irritation documented.
According to a different study, the Eversense CGM system maintained a satisfactory safety profile for patients with type 1 and type 2 diabetes while providing accurate glucose readings for the duration of the sensor’s planned 90-day life.
Read Next New Integrated Continuous Glucose Monitor Developed: A Breakthrough in Diabetes Care
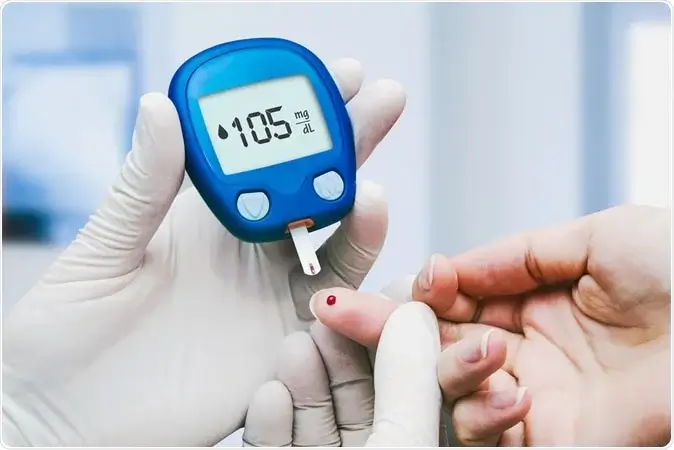
Do You Need to Do Fingersticks If You Use a Continuous Glucose Monitor (CGM)?
According to Pouya Shafipour, MD, a board-certified family and obesity medicine physician at Providence Saint John’s Health Center in Santa Monica, California, patients should always have a backup technique for monitoring their blood sugar levels, even if they are using a Continuous Glucose Monitor (CGM) device.
Since these gadgets are still in their infancy, mistakes can still happen. Things like heat, food, and supplements might affect them, so it’s always a good idea to have a backup plan, like a fingerstick, on hand, according to Shafipour.
Fingersticks are not necessary for people without diabetes or even for those with prediabetes. He did, however, caution people against accepting Continuous Glucose Monitor (CGM) readings as gospel.
“People should be coached to understand that the numbers might not always be 100% accurate,” Shafipour said, adding, “Based on my experience, there can be errors with these.”
Blood Pressure Trackers That Don’t Need a Finger Prick
What Perils Are Associated with Implanted CGMs?
Although CGMs have several advantages, Shafipour noted that they may also result in infections or skin irritation at the site of the sensor insertion. Although these instances are uncommon, there is a small chance that the body will reject the device.
Inaccurate readings from CGMs can also result from body temperature fluctuations or the influence of specific foods, drugs, and supplements.
When Will It Be Possible to Get the Eversense 365 CGM?
Eversense 365 will soon be available in the United States, according to the creators, who anticipate beginning delivering by the end of October. Patients who are interested in the gadget can pre-register on their website, according to Hansen.
Commercial insurance provides extensive coverage for Eversense. According to Hansen, “We anticipate that most commercial health plans will cover Eversense 365, and we are constantly working to broaden access as widely as possible to the product’s unique benefits.”