Diabetes management is undergoing a significant transformation with the FDA’s recent approval of implantable continuous glucose monitors (CGMs) that can integrate with insulin pumps. This development marks a pivotal moment in diabetes care, providing patients with more precise, automated glucose control. In this blog, we will explore the implications of this advancement, how it works, and what it means for individuals using insulin pumps. By understanding the benefits of this technology, patients and healthcare providers can make more informed decisions about diabetes management.
Insulin Pumps’ Function in the Management of Diabetes
Insulin pumps have been a critical tool for individuals with diabetes, particularly those with Type 1 diabetes. These devices deliver insulin continuously through a catheter placed under the skin, mimicking the body’s natural insulin release. Insulin pumps offer the advantage of precise insulin delivery, reducing the need for multiple daily injections and helping maintain stable blood glucose levels.
However, managing diabetes with an insulin pump still requires regular monitoring of blood glucose levels. Traditional methods, such as fingerstick tests, can be cumbersome and often fail to provide a complete picture of blood glucose trends. This is where continuous glucose monitors (CGMs) come into play, offering real-time data that can significantly improve diabetes management.
What Is an Implantable CGM?
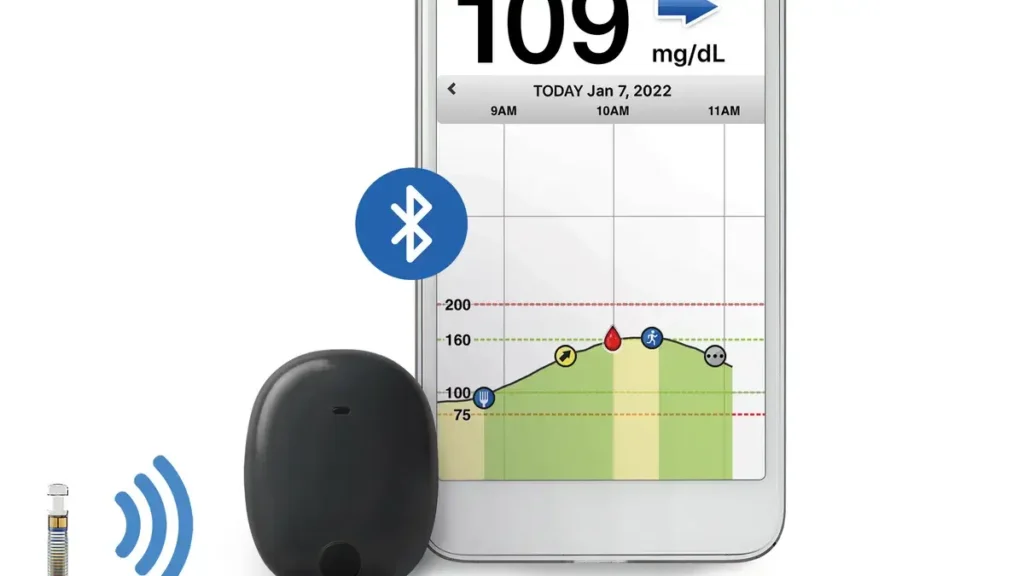
A continuous glucose monitor (CGM) is a device that tracks glucose levels in real time, typically through a sensor placed on the skin. By implanting the sensor beneath the skin, an implantable continuous glucose monitor advances this technique. This type of CGM offers several advantages over traditional CGMs, including longer wear times and reduced discomfort.
With the FDA’s recent approval, implantable CGMs can now be integrated with insulin pumps, creating a seamless system that continuously monitors glucose levels and adjusts insulin delivery accordingly. This integration represents a significant leap forward in diabetes care, as it allows for more precise glucose management with minimal patient intervention.
FDA Approval and Its Impact on Diabetes Care
The FDA’s decision to allow the integration of implantable CGMs with insulin pumps is a game-changer for diabetes management. This approval means that patients can benefit from a more automated and personalized approach to insulin delivery, reducing the risk of both hyperglycemia (high blood sugar) and hypoglycemia (low blood sugar).
The integration of these technologies enables what is often referred to as a “closed-loop” system or an “artificial pancreas.” In this system, the implantable CGM continuously monitors glucose levels and sends data to the insulin pump, which then adjusts insulin delivery based on real-time readings. This reduces the burden on patients to manually check glucose levels and adjust insulin doses, leading to better overall glucose control.
More Read About How Sensors and Insulin Pumps Connect Diabetes Care?
Benefits of Integrating Implantable CGMs With Insulin Pumps
There are numerous important advantages to integrating insulin pumps and implanted CGMs:
Improved Glucose Control
The real-time data provided by the CGM allows for more precise insulin delivery, helping to maintain glucose levels within a target range.
Reduced Risk of Hypoglycemia
With continuous monitoring and automatic insulin adjustments, the risk of hypoglycemia is significantly reduced.
Increased Convenience
Patients no longer need to perform multiple fingerstick tests or manually adjust insulin doses, making diabetes management more convenient and less intrusive.
Longer Sensor Life
Implantable CGMs typically have a longer lifespan compared to traditional CGMs, meaning fewer sensor changes and less disruption to daily life.
Enhanced Quality of Life
The integration of these technologies can lead to better overall health outcomes and an improved quality of life for individuals with diabetes.
Challenges and Considerations
While the integration of implantable CGMs with insulin pumps offers numerous benefits, it is important to consider the challenges and potential drawbacks as well.
One of the primary concerns is the cost associated with these advanced technologies. Implantable CGMs and insulin pumps can be expensive, and not all insurance plans may cover them. Patients should work closely with their healthcare providers to understand the costs and explore available financial assistance programs.
Another consideration is the learning curve associated with using new technology. While these systems are designed to be user-friendly, there may be a period of adjustment as patients and healthcare providers become familiar with the new devices. Proper training and support are essential to ensure successful use.
Additionally, there may be concerns about the accuracy and reliability of the implantable CGMs, particularly in the early stages of adoption. However, the FDA’s approval process includes rigorous testing to ensure that these devices meet high standards of safety and efficacy.
The Future of Diabetes Management With Insulin Pumps
The FDA’s approval of implantable CGM integration with insulin pumps is just the beginning of a new era in diabetes management. As technology continues to evolve, we can expect to see even more advanced systems that offer greater precision, ease of use, and personalization.
One area of potential growth is the development of fully closed-loop systems that require minimal patient input. These systems could automatically adjust insulin delivery based on real-time data, taking into account factors such as physical activity, stress, and diet. This would allow for truly individualized diabetes management, reducing the burden on patients and improving health outcomes.
Introducing artificial intelligence (AI) into these systems is another exciting direction. AI could analyze vast amounts of data from the CGM and insulin pump, providing insights and recommendations to further optimize glucose control.
Conclusion
The FDA’s approval of implantable CGMs that can integrate with insulin pumps marks a significant milestone in diabetes care. This advancement offers patients a more automated and personalized approach to managing their condition, potentially improving glucose control and overall quality of life.
While there are challenges to consider, such as cost and the learning curve associated with new technology, the benefits of this integration are clear. As we look to the future, continued innovation in diabetes management holds the promise of even more effective and convenient solutions for individuals living with diabetes.